Anodizing at Home, Using Sodium Bisulfsate Instead of Sulfuric Acid.
Aluminum (along with most other metals) naturally grows a passivation layer of metal oxide. This protects the underlying metal from corrosion. That is why your car/house/other metal object doesn’t dissolve in the rain.
Unfortunately this layer is very thin- only a few nanometers. A tiny scratch ruins it. Once the oxide layer is breached, it’s a free for all as various chemicals in the local environment rush in to eat away that delicious exposed metal.
Using simple means, this natural oxide layer can be grown thicker- several micrometers thick. This is an electrochemical process better known as anodization. Anodized aluminum has unique properties that are highly desirable. If you work with aluminum, you’ll want to anodize stuff sooner or later.
I’ve been wanting to experiment with anodization for a while now. I had some trouble finding sulfuric acid, which prompted me to search for alternatives. While I can’t guarantee the following process is identical to sulfuric acid anodization, it does appear to work okay for small projects.
Sodium Bisulfate Anodization
Anodization is typically accomplished with a direct current source and an acid bath. Direct current is easy. You’ll have no trouble finding DC power supplies or batteries. It’s the acid bath that’s problematic.
Industry standard is sulfuric acid (H2SO4). You can get sulfuric acid sometimes. Chemicals are one of those things that are inconsistently regulated. It all depends on where you live, what headline-grabbing hysteria is gripping the nation, and whether anyone wants to ship hazardous materials.
Either way, sulfuric acid is nasty stuff. It can easily cause life-changing injuries, eats through most common materials, and reacts in all kinds of unpleasant ways- including with water. There’s another way.
Sodium bisulfate (NaHSO4) is an acid salt formed by the partial reaction of sulfuric acid and a sodium base. Chemically speaking it’s similar to sulfuric acid. In more practical terms, it’s a mostly unregulated chemical used as a pH reducer. It comes in solid form which is significantly less hazardous. You can buy loads of it in the form of pool acidifier.
I can’t take credit for this. I found it over here. Sadly the original author (Ken) died June 2021. Thanks Ken, you made an intimidating process much more approachable. In the interest of keeping this knowledge available, I’m going to go into a lot more detail than I normally would.
Preparation
Before going any further, I need to say this is dangerous. I’ll do my best to point out the hazards, but I can’t predict everything that could go wrong with any particular setup. You do this at your own risk.
I am not a chemist. The last time I did chemical experiments was about ten years ago. I can’t explain jack shit here. Don’t use me as an authoritative source; be prepared to do supplementary research.
If you’re prepared to accept that responsibility, read on.
If you won’t accept that responsibility, stop reading and go do something else.
Lab Safety
Before starting, it’s a good idea to review chemical safety protocol. The chemicals involved are relatively easy to get your hands on, but that doesn’t mean they aren’t dangerous!
Read through the material safety data sheets for sodium hydroxide and sodium bisulfate. Might as well read the sulfuric acid one too, just in case.
Splash proof goggles are mandatory. Gloves are optional but recommended. They prevent harsh chemicals from reaching your skin, and they prevent skin oils from reaching the workpiece.

Splash goggles make a tight seal around the eyes. They don’t usually have vents.
Any rubber gloves should work- these are latex cleaning gloves.
Do not even think of eating or drinking around the lab area. You’ll contaminate the chemicals and/or poison yourself. Oh, and keep any pets, children, and irresponsible sorts away. You can let them back in once everything is cleaned up and put away.
Gas bubbles are formed during the process, so be prepared to deal with splatter. Don’t lean over the anodization bath, and keep the room ventilated.
Finding Supplies
Water- Any clean, fresh water should work. Distilled water is preferable, but soft tap water can be used. Hard water should not be used.
Sodium Hydroxide- Easily obtained as drain cleaner. Look for “100% Lye” or similar. Don’t use anything with extra stuff added in.
Sodium Bisulfate- Available as pool “ph down” wherever pool chemicals are sold. Easily found online. Also available as a food additive. Just make sure it’s not mixed with anything else.
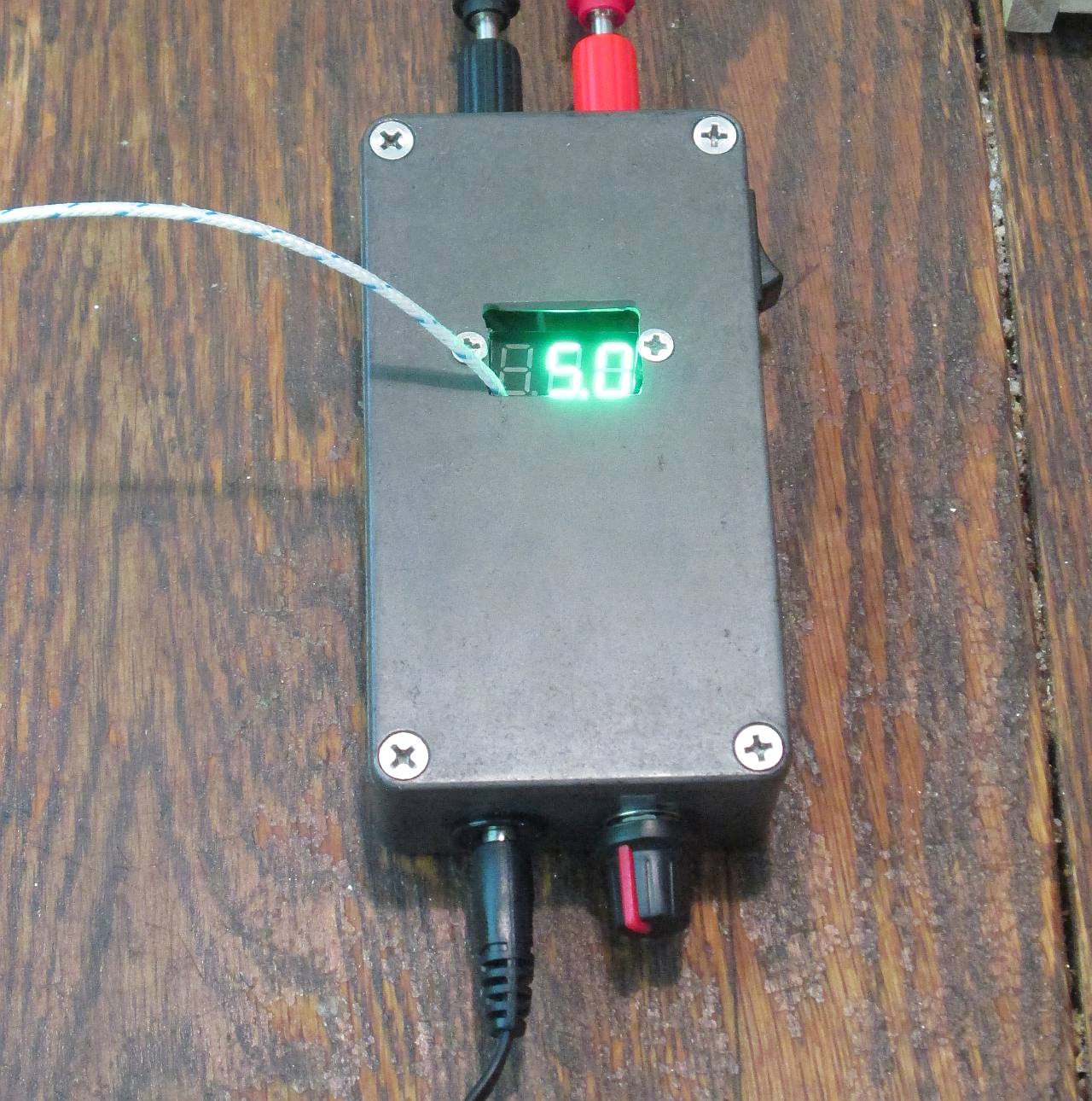
Power Supply- A constant-current power supply capable of putting out about 12V at 2A is enough for a small part. Standard bench supplies should work, but be wary of running them to their limits. Alternatively, build your own. Batteries can be used in a pinch.
Containers- Most plastic containers will work. Look up the chemical compatibility of the plastic if you’re unsure. Use a thick, tough container that can take some abuse.

Sodium bisulfate, lye, black dye, and fresh water.
Also some plastic sheet to protect the table and a plastic stirring stick.
Disposal
Disposal is straightforward. All the chemicals involved can be poured down the drain with a clean water chase. After all, sulfuric acid and sodium hydroxide are sold as drain cleaner.
However, it’s always a good idea to neutralize them. Simply drip a weak acid/base into the solution until it stops reacting. Vinegar and baking soda will do the job. These neutralizing solutions are also useful for cleaning up spills.
The 720 Rule
Calculating the thickness of the oxide layer is pretty easy- once you find the equation. Search for the “720 Rule”. In short:
m =\frac{t \times 720}{I \div A} [\latex]
- m- Minutes to anodize
- t- Oxide thickness in thousandths of an inch
- I- Current in amperes
- A- Area in square feet
Just plug and chug.
For people who use sensible units of measurements, it’s the “312 Rule”:
m =\frac{t \times 312}{I \div A} [\latex]
- m- Minutes to anodize
- t- Oxide thickness in micrometers
- I- Current in amperes
- A- Area in square decimeters
While the units in these equations aren’t always convenient, conversions are straightforward. I’d strongly suggest putting it all in a spreadsheet.
Note that these equations assume a constant current. Constant voltage does not work the same way, and is much harder to get right.
Anodizing Process
Remember the ugly aluminum case I used for Simple Power? I mentioned anodizing in passing. Well, it’s about time I did something about that. It’ll make a good piece to illustrate the anodizing process. If I screw it up, no problem.

I still insist this is the blandest thing imaginable.
There are five steps to the process, two of which are optional. If you’re playing along at home, read through the entire process before you start.
Clean
First, the metal must be cleaned. Clean it with soap, water, and a scrubber. Rinse thoroughly. Surface contamination will cause trouble down the line. Don’t handle the cleaned part with bare hands unless you want smudges permanently burnt into it.


Rinse the cleaned part thoroughly and let it dry. Proceed to the next step.
Etch
Etching the metal improves the process. By far the easiest way to do this is using sodium hydroxide. Only a dilute solution is required- 20g/L (2%) is fine. After a few minutes, the part is withdrawn from the solution and rinsed.
As a general rule, mix chemicals into water, not the other way around. Add them together slowly. You can get a steam explosion if there’s not enough cold water to absorb the dissociation energy.
You ARE wearing your goggles, aren’t you?


While it has some nice effects, etching is optional. You can safely skip this step.
Anodize
Next, the metal is put in the electrolysis bath. This is a relatively strong solution of sodium bisulfate- 250g/L (20%). Keep any non-aluminum metals out of the bath. They’ll dissolve and contaminate it.
Oxygen is produced at the anode (hence anodization), so the part is connected to the positive terminal. The cathode should be aluminum. Cathode area should be at least as big as the part. Make it surround the part as much as possible.
Any areas of the metal that need accurate dimensions (e.g. screw threads) should be masked off. You can get special electroplating tape but my hunch is any plastic tape would work. Plastic screws are ideal for plugging up internal threads.
Suspending the case in the anodization bath was a bit tricky. I don’t have any aluminum clamps, fasteners, clips, or wire. After some serious thought, I settled on this:

Note the plastic screws holding the lid on.
Mounting hardware will leave small un-anodized areas where it’s attached. This unorthodox contraption puts them under the lid, out of sight.
My calculations show it’ll take about 40 minutes for the case to grow a 10um layer. I have no way to measure the thickness, so I just have to take it on faith and trust the process.
Note that the anodizing process is not self-limiting. You’ll have to pull it out after the time is up.
Anything over 25um is considered “hard anodized” and requires better equipment, so stay under 25um for best results. 5-10um ought to be enough for most purposes.

Hooked up, with the full 2A flowing through.

Look at all those bubbles!

Once the anodizing is finished, pull the part and rinse it immediately. Get into any holes and crevices. You don’t want to leave any trace of acid on the part.
Color
Natural anodization color ranges from faint gray, to straw yellow, to dark green and black. It depends strongly on oxide thickness and the particular alloy being anodized.
If you want a specific color, you can add dye before sealing. Most colors work, except white. I chose to use black dye at a concentration of 10% (100mL/L). Just let it sit until it’s dark enough.

Dying was done in the sink, because this stuff will stain anything it touches. I threw the aluminum strip in, just to see what would happen.

Dying is, of course, optional. Feel free to skip straight to sealing.
Seal
Fresh out of the anodization bath, the oxide layer has lots of tiny pores. These let the acid bath in to continue the process, but they will also let in everything else. Sealing is mandatory to protect the part.
Sealing is surprisingly easy. Boil the part in water for 20 to 30 minutes. Steam works too, if that’s easier.

Adding hot water turns oxide into hydrated oxide. This is physically bigger so it seals up the pores. It’s not perfect, so an additional layer of sealant on top helps. Any clearcoat that sticks to metal is fine. Feel free to put a coat of paint on top- anodized surfaces take paint just fine.
Results
When I started this process, I had no idea whether my box would anodize properly. Some sources said yes. Some said no. I don’t care too much if I ruin this monument to blandness, so might as well give it a go.

Success! Partly! Partial success!
There are a few problems that need to be worked out.
Close inspection shows uneven surface finish. In particular, there’s these strange dark spots:


I’ll assume for now this was the result of inadequate cleaning. I didn’t spend too much time on it, and paid a price for it later. Uneven field distribution in the bath might also have a role to play.
The other problem was getting the dye dark enough. I left the metal in the dye for a few hours, but it never got darker than this. My best guess is the oxide layer simply wasn’t thick enough. Perhaps I should have anodized for a full hour.
Neither of these problems makes the case unusable, merely aesthetically unpleasing. It still looks better than when it was bare metal. For less utilitarian applications, this would be unacceptable. I’ll need to do some more work on the process before taking on something more ambitious.
Finishing Up
Anodizing is something I wanted to try for a long time now. Discovering the sodium bisulfate method was key- sulfuric acid is just too hard to get these days. I’m also less inclined to keep sulfuric acid around. When you’re young you don’t always appreciate the risks you’re taking.
I’m happy to say that the bisulfate process does in fact work. There are a few minor problems to work out, but they shouldn’t be unsolvable. I’ll be perfecting the process for a while yet.
Anodizing solution can be reused multiple times. I don’t know when (if?) the bath ever “wears out”, but you can get a few parts done without having to mix new chemicals each time.
Holding the workpiece turns out to be the most complicated part. You can’t use many materials. Get creative. Plenty of more complex ideas were passed over on the way here, some of which I might revisit.
Other acids can be used, but it’s not a trivial replacement. Some acids work, some burn huge holes in the metal. Doubtless some do far, far worse. I doubt any of them will be easier to get than sulfuric acid though, so what’s the point?
Process control is required for thick anodization. This includes temperature control, higher voltages, higher current, and possibly different chemicals. I might look into this, but the chemical engineering involved is beyond me at the moment.
Scalability is good, though a large piece requires a larger bath. More current too. Temperature control starts to become a concern with larger pieces and longer run times. Running current through water heats it up; large anodization baths typically need cooling.
Anodizing has been an on-and-off project. It took a few years to get the chemicals, power supply, and a suitable test piece together. I was worried that any part of the process could go horribly wrong, but in the end it was a smooth operation from start to finish.
Of course, the limitations of my equipment were made apparent. I’ll need a better power supply. Bigger containers too. Lots of new ideas are bouncing around my head- I’ll save them for next time.
Have a question? Comment? Insight? Post below!